Like Mini-Med, Animas’ first product to market was an insulin pump, but they are also developing an interesting long-term implanted glucose sensor. Based in West Chester, Pennsylvania, and founded by engineer Katherine Crothall, Animas is putting together the ingredients for an artificial pancreas. Ultimately this sensor will be tied into an insulin infusion pump to provide closed-loop control of blood glucose levels.
The Glucose Sensor
Animas’ sensor has been designed from scratch for accuracy and for long-term(> 5 years) internal use. The Animas sensor is based on Spectroscopy. They decided not to base their sensor on enzyme systems such as those used by MiniMed, Cygnus, and others which have inherent problems of drift hydrogen peroxide production, and fibrous buildup that interferes with accuracy. These problems require users to perform frequent calibration with a standard meter. The Animas sensor has the advantage of being able to directly read glucose in the blood.
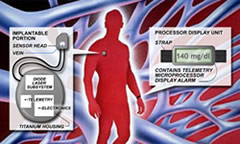
In contrast to the fumblings of external near-IR manufacturers like BioContol and Futrex, the Crothall team miniaturized their sensor to minimize scatter problems found in their big brethren. They then hypersensitized the receiver to pick up the very tiny changes in glucose levels found within a small blood vessel. A small, ultralight C-clamp detector is surgically implanted around a 4 to 5 millimeter (0.2 inch) diameter blood vessel. The detector has two tiny probes at the tips of the C-clamp structure which puncture each side of the vessel and allow transmission of a clean infrared light signal between them.
A larger device housing a laser generator plus signal analysis is located nearby within a closed compartment under the skin. The laser IR signal is transmitted to the detector around the vessel and returns the transmitted beam back to the processing unit. This approach appears to be working well with a reported R-squared accuracy of 0.94, compared to 0.72 for the GlucoWatch and 0.64 for MiniMed’s 3-day CGMS external sensor.
A major advantage to this approach is that calibration would be required only once a week and they are attempting to develop a system requiring no calibration. The laser system is also much less prone to interference from the buildup of fibrous membranes or scarring compared to enzyme-based systems. Although minor surgery is required, it provides direct access to blood rather than measuring glucose in the surrounding interstitial fluid.
The larger device housing the battery and laser generator is described as being twice the size of a pacemaker. If a rechargeable battery can replace the static battery in the device and this is likely, the size could be cut in half. Readings would be available every 2 to 5 minutes.
The Glucose Sensor is not yet available. They are now in the process of designing a miniaturized sensor that is in preliminary ex-vivo human trials since late 2003.
Last Updated on October 15, 2019

