Due to complaints about glucose meter inaccuracy, the Food and Drug Administration (FDA) is currently developing a new standard for glucose meter accuracy. This update is driven by ongoing improvements in meter accuracy, the need for better accuracy with today's pumps, bolus calculators, and continuous glucose monitors, reports of hospital and out-patient deaths, consumer complaints, and research studies showing that several approved glucose meters failed to meet FDA or International Standards Organization (ISO) standards in post-approval testing.1-3
Most users assume their glucose meter is accurate and use it to make therapeutic decisions. Yet significant differences in accuracy exist between and within meter brands that are often unrecognized by clinicians and people with diabetes. For example, brand-to-brand variations may be discovered when a health insurer selects a lower cost meter as the preferred brand and user perform simultaneous glucose tests on the old and new meters. The greatest danger that the FDA is concerned about occurs when an erroneous reading on a glucose meter causes severe hypoglycemia or death after an excessive dose of insulin in a hospital or home.
What Is Accuracy?
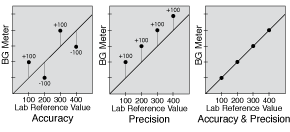
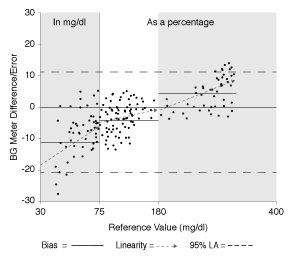
The term “accuracy” used in this paper in relation to glucose meters is an inclusive term that includes all aspects of accuracy. These aspects include accuracy and precision, shown in Figure 1, as well as a low bias, close linearity, and a narrow limit of agreement as depicted in Figure 2. Optimal glucose meter accuracy can be thought of as a minimum of total error.
Numerous glucose meter factors can distort accuracy. These include meter error, test strip manufacturing defects, test strip lot variations, underfilling of test strips, glucose meter time errors, environmental factors (temperature extremes, humidity, and high altitude), plus a variety of other factors (anemia, hypoxia, being on oxygen therapy, dialysis, and various medications). Other factors beyond glucose meter errors can complicate the path to great readings, such as errors in carbohydrate counting or insulin dosing, varied activity, and others. Any error introduced into insulin dosing decisions adds to the propagation of total error.
Accuracy Issues
The FDA reported 100 deaths associated with potential glucose meter inaccuracies between 1992 and 2009 and 12,672 serious injuries from 2004 to 2008.(4) Richard Hellman, M.D., past president of the American Association of Clinical Endocrinologists (AACE), responded to a New York Times editorial regarding glucose meter accuracy, saying, “There is considerable concern from many quarters that the lower-than-optimal accuracy of glucose meters in current use, both in the inpatient and outpatient setting, is a cause of errors in insulin dosage, with resultant morbidity, and possibly mortality.”(5)
Why Do Glucose Meters Need To Be More Accurate?
Today’s technology demands greater glucose meter accuracy. For example, an insulin pump can accurately deliver insulin in doses of 0.025 U or less, with a precision of the order of 0.06% for someone who uses 40 U/day. This precision is significantly compromised when the glucose meter used to determine insulin doses varies in accuracy by ±20%. With a target glucose of 100 mg/dl, a pump’s bolus correction calculator cannot safely lower readings higher than 200 mg/dl when 95% of the meter’s BG readings are allowed to range between 160 and 240 mg/dl, and 5% of readings may be outside this range.
Thus an accurate insulin correction dose for a glucose meter reading of 200 mg/dl may produce a glucose outcome between 60 and 140 mg/dl. As glucose values rise above 200 mg/dl, accurate correction doses based on a relatively inaccurate glucose meter will create ever-increasing risks for hypoglycemia or ongoing hyperglycemia in spite of very accurate dosing by the pump. Most meters are much more accurate than this, but this is the current accuracy allowed for meter approval.
Even if the FDA tightens their standard to the proposed 2012 ISO standard of having an accuracy within ±15% above 75 mg/dl, insulin dosing errors with intensive glucose control are still likely. With this standard, a glucose meter reading of 300 mg/dl can actually be a plasma value between 255 and 345 mg/dl. After insulin is accurately delivered to correct the “300 mg/dl” reading, the final glucose may end up anywhere between 55 and 145 mg/dl on 19 out of 20 readings.
All current commercial CGM devices are calibrated from glucose meter readings. Research shows that CGM accuracy is greatly improved when a CGM device is calibrated from laboratory venous plasma glucose levels rather than glucose meter readings.(6) Accurate calibration of CGM devices has become more and more important because wearers use their CGM readings to calculate insulin doses even though this is not FDA approved.
Meter errors are magnified in the real world because glucose meter accuracy is decreased when alternate test sites are used,(7) and when more than one brand of meter is used, where bias may vary by as much as 30% to 40%. Bolus calculators that correct for insulin stacking are common in insulin pumps. They are also becoming more widely available in meters and phone applets for those who use insulin pens and injections. This increases the need for meter accuracy for more and more people using insulin.(8)
Need For Post-Approval Testing
Several studies have revealed a need for ongoing evaluation of glucose meters after they have received FDA or ISO approval. One review of 27 meters that had previously been approved with the 2003 ISO 15197 standard, only 16 of them actually met this standard in post-approval testing.(1) Another study found three of nine meters failed the ISO standard when testing was performed by patients,(2) and another study revealed that when people used 21 different glucose meters, 16% of their measurements were more than 20% above or below the lab value, far exceeding the recommended 5% or less in the ISO and FDA standards.(3)
Steps The FDA Can Take
The accuracy of any meter needs to be matched to what it will be used for. Someone with type 2 diabetes who uses diet only or medications with little risk of hypoglycemia would not require a meter that is as accurate as someone who is pregnant, someone who users insulin to maintain tight glycemic goals, or a critically ill patient in a hospital who is being treated with tight glycemic control protocols.
Glucose meters are being used in increasingly diverse situations, so today’s meter accuracy needs to be matched with their specific clinical uses. These include intensive glucose control with a bolus calculator, pregnancy, use in a child who is unable to communicate hypoglycemia symptoms, those with hypoglycemia unawareness, calibration of a CGM device, those with type 2 diabetes treated with diet or oral agents, and for hospital use. Such intended-use gradients would ensure that each glucose meter matches the user’s or hospital’s need for accuracy.
Currently, the FDA gives a yes or no approval for meters that are submitted. In a new paradigm, meters that receive approval using a more accurate standard such as +/-15% for 97% of readings would be further graded in terms of accuracy. Each meter’s accuracy would be published by the FDA and clearly shown on meter and test strip containers. Ideally, a panel of diabetes inpatient and outpatient clinicians would be formed to recommend the accuracy requirements for different groups. This would allow those buying test strips and their health insurers to know that the meter being used actually meets the user’s clinical needs. Yearly or biyearly retesting of the meter with at least 3 lots of test strips purchased from pharmacies or distributors will also be needed to ensure accuracy is maintained over time in the manufacturing process.
These and many other suggestions to improve meter accuracy and reduce clinical error are reviewed in recent publications.(9,10)
- Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, Heister F, Haug C. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;12(3):221–31.
- Kristensen GB, Monsen G, Skeie S, Sandberg S. Standardized evaluation of nine instruments for self-monitoring of blood glucose. Diabetes Technol Ther. 2008;10(6):467–77.
- Alto WA, Meyer D, Schneid J, Bryson P, Kindig J. Assuring the accuracy of home glucose monitoring. J Am Board Fam Pract. 2002;15(1):1–6.
- Harper Lias CC. Presentationat FDA/CDRH public meeting. Blood glucose meters. March 16 and 17, 2010, Gaithersburg, MD.
- Hellman R. AACE patient safety – editorials: commentary on July 19 New York Times article on glucose monitor accuracy.
- Kamath A, Mahalingam A, Brauker J.Analysis of time lags and other sources of error of the DexCom SEVEN continuous glucose monitor. Diabetes Technol Ther. 2009;11(11):689–95.
- Kilo C Sr, Dickey WT Jr, Joynes JO, Pinson MB, Baum JM, Parkes JL, Parker DR. Evaluation of a new blood glucose monitoring system with auto-calibration for home and hospital bedside use. Diabetes Res Clin Pract. 2006;74(1):66–74.
- Walsh J, Roberts R, Bailey T. Guidelines for optimal bolus calculator settings in adults. J Diabetes Sci Technol. 2011;5(1):129–35.
- Walsh J, Roberts R, Vigersky RA, Schwartz F. New Criteria for Assessing the Accuracy of Blood Glucose Monitors Meeting, October 28, 2011. Journal of Diabetes Science and Technology 2012; 6(2), 460-468.
- Heinemann L, Lodwig V, Freckmann G. Accuracy in Blood Glucose Measurement: What Will a Tightening of Requirements Yield? Journal of Diabetes Science and Technology 6(2), 2012; 435-443.