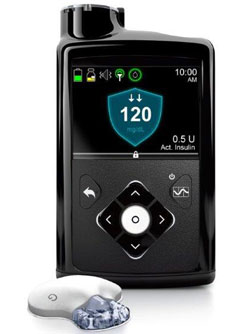
Minimed received FDA approval for its 670G system in September 2016. Expected to ship in Spring of 2017, the 670G automatically adjusts the delivery of your basal insulin based on readings from Minimed's new CGM, the Guardian Sensor 3 and the Guardian Link 3 transmitter. The sensors are said to have a 7-day life span while being continuously monitored by their new diagnostic technology that monitors how the sensor is doing. This is currently the only sensor approved to drive the amount of insulin delivered by a pump.

Minimed received FDA approval for its 670G system in September 2016. Expected to ship in Spring of 2017, the 670G automatically adjusts the delivery of your basal insulin based on readings from Minimed's new CGM, the Guardian Sensor 3 and the Guardian Link 3 transmitter. The sensors are said to have a 7-day life span while being continuously monitored by their new diagnostic technology that monitors how the sensor is doing. This is currently the only sensor approved to drive the amount of insulin delivered by a pump. You must still enter carbs when you eat and blood glucose (BG) readings to calibrate the sensor, but automatic adjustments of the basal insulin means there is a lot less input needed compared to current systems. For those who are not quite ready for Minimed's Auto Mode, you still have the Suspend on Low and Suspend Before Low modes that allow you to set low limits for the pump to stop insulin delivery.
If you want to be first in line to receive the new system, Minimed has a Priority Access program that allows anyone who purchases a MiniMed 630G system between August 11, 2016 and the ship date of the MiniMed 670G system to upgrade for $299.
Take a look at the full Minimed 670G press release.
