DexCom, headquartered in San Diego, California, develops continuous glucose monitoring technology that improves the lives of people with diabetes. The FDA approved their first DexCom STS system on March 27, 2006. Ongoing upgrades to their technology have now produced the Dexcom G4 Platinum (now up to the G7) that is considered to be the most accurate CGM system at this time.1,2 Dexcom Share, basically a bedside device that not only charges the G4 but also transmits the user’s glucose readings from a nearby iPhone to any other iPhone, was approved on Oct. 20, 2014.
The Gen 4, approved for use in children aged 2 and older, can be used by placing the sensors on the upper buttocks or abdomen of the child. However, parents are cautioned not to rely solely on the CGM for readings.
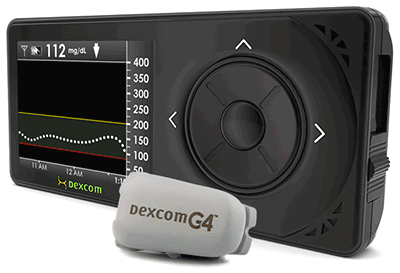
The Dexcom G4 Platinum ships with:
- The G4 Platinum Transmitter – discreet, wireless transmission up to 20 feet, gentle adhesive
- The G4 Platinum Receiver – 4″ x 1.8″ x 0.5″, color screen, new shape, stores 30 days of data, data is downloadable to a computer
- USB charging cable and charger
- receiver carry case/belt clip
- Dexcom Studio Software
- User’s Guide
- Tutorial Disc
The Dexcom G4 features a tiny, flexible wire sensor that is inserted under the skin through a 26-gauge needle, which is easily inserted with a disposable applicator. The design of the applicator allows nearly painless insertion of the needle that the applicator withdraws, leaving behind the wire sensor under the skin. Sensors are sold separately from the monitor in packs of 4.
Additional features:
- Trends are shown in hues of yellow, red, and white for quick recognition of highs, lows, or good readings
- Custom tones and melodies are available for high or low alerts
- Receivers come in Classic Black, Tickled Pink, and Ocean Blue
Dexcom is working with Animas and Tandem insulin pump companies to move the CGM display onto the pump screen, both of which are in color. Asante and Omnipod will work with Dexcom’s G5 device in the future, which will transfer CGM readings to smartphones using Bluetooth Low Energy. For pump wearers, these approaches eliminate the readout device, although two skin sites are still required, one for the glucose sensor and another for the infusion set.
Dexcom Studio Software
Dexcom Studio software allows you to view and analyze readings collected from your Dexcom receiver. Connect your receiver to your computer using the enclosed USB cable to launch Dexcom Studio and retrieve any new readings. The receiver can hold up to 30 days of sensor data. Once complete, the oldest records are deleted to make room for new readings. Plan to upload your data every 3-4 weeks to ensure all sensor readings are included in your charts and reports, rather than being lost. Dexcom Studio is currently compatible with Windows but does not run on a Mac.
Dexcom Studio features include:
- Dexcom Portrait Report -Summary statistics of overall glucose control with clinical assessment notes.
- Hourly stats – All-in-one report to easily assess glycemic patterns and variability at the same time.
- Daily trends – Overlapping days to assess glycemic patterns.
- Glucose Distribution – Shows percent time high, low, and in target glucose ranges and overall glucose distribution. Assess pre- and post-prandial control by comparing the pie chart distribution.
- Glucose Trends – Report to review daily glucose trends, event markers, and correlation between SMBG and CGM. Daily Strip Print feature prints daily glucose trends with a touch of a button.
- Daily Stats – Review percent time spent high, low, and in target glucose ranges by day.
- Success Reports – Compares glycemic control weekly, monthly, or quarterly.
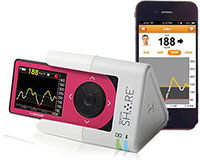
Dexcom Share
The Nightscout Project has demonstrated that CGM data can be shared in real time with others. Dexcom took the next step by obtaining FDA approval for the Dexcom Share device in October 2014, following its submission to the FDA for approval in July 2013. Share is the first FDA-approved device that allows CGM readings to be remotely viewed, and promises to be a great help to parents, partners, and physicians of people with diabetes.
Share is a cradle-like device that you plug your Dexcom receiver into. The cradle, however, must be plugged into an electrical outlet to transmit your data via Bluetooth to the Dexcom Share App on an iPhone (4S or later) or iPod Touch (5th generation or later). You may only pair one device with the cradle at a time. The system is not yet available for Android phones. Readings can then be sent from the cradle to the cloud and be accessed by a family member or friend’s iPhone or iPod once they add Dexcom’s Follow App and once you permit them to do so. The Share app must be running in the foreground or background to receive data. You can invite up to 5 people to receive CGM data from your Share device. The received data is completely programmable, allowing those who receive your data to choose exactly which information they would like to be notified about.
The Share sells for $299, which is less than one-tenth the cost of Medtronic’s mySentry remote CGM receiver, which does not offer Bluetooth or cloud connectivity. You do not need a prescription for Share, but please note that insurance companies are not yet covering the device, so you will need to pay out of pocket. Refer to the Dexcom Share FAQ for more information.
1 V. Matuleviciene, J.I. Joseph, M. Andelin, I.B. Hirsch, S. Attvall, A. Pivodic, S. Dahlqvist, D. Klonoff, B. Haraldsson, and M. Lind. A Clinical Trial of the Accuracy and Treatment Experience of the Dexcom G4 Sensor (Dexcom G4 System) and Enlite Sensor (Guardian REAL-Time System) Tested Simultaneously in Ambulatory Patients with Type 1 Diabetes. http://online.liebertpub.com/doi/full/10.1089/dia.2014.0238
