The Glucoday S is a continuous monitor made by A. Menarini Diagnostics. The Glucoday S inserts a microfiber in your abdomen that uses microdialysis to monitor blood glucose levels. Just the size of a portable audio device like a Walkman tape player, this device features:
- a one point calibration per 48 hour monitoring period
- wireless real-time display on instrument and pc
- blind or displayed mode operation,
- alarm (buzzer or vibration) in case of hypoglycemia or hyperglycemia
- less than 2 min delay in response time,
- long life onboard biosensor (up to 6 months after the first use at room temperature) and an accessible data base.
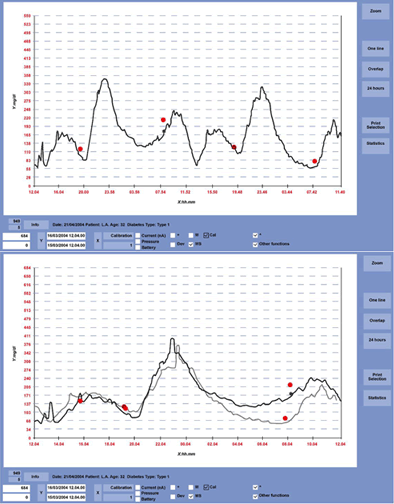
The GlucoDay® S is specifically for clinical use. It is worn by the subject for a 48 hour period. After calibration of the device to the patient, a physician would then insert the microdialysis system into the patient. Going about their normal activities, GlucoDay® S records realtime glucose values for analysis by a health care team. It produces charts like the ones shown to the right.The main goal for the device is to chart an individuals daily glucose activity especially during sleeping hours. With this information the health care team and the patient can modify the insulin regimen in order to achieve better control patterns.
The GlucoDay® S is available for purchase in Austria, Belgium, Italy, France, Germany, Greece, the UK, Portugal, Spain, and the Netherlands. No release date for the US has been announced.
About A.Menarini Diagnostics
A.Menarini Diagnostics is a health care company with a worldwide network of affiliates, partners, and distributors. They have been in the pharmaceutical business for over 100 years, and in the last 30 years has become Italy’s largest pharmaceutical R&D company. They released the GlucoDay® S semi-invasive continuous glucose monitor, which is the first system based on a “microdialysis” technique in the world to obtain the CE marking for the Medical Device Directive, which is necessary for the marketing of a product in the European Union.
Last Updated on June 13, 2020