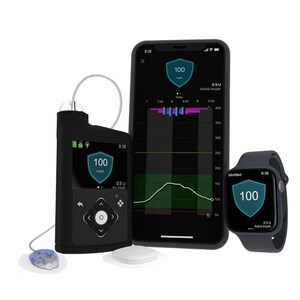
The MiniMed 780G System is an advanced automated insulin delivery (AID) device designed to simplify diabetes management for individuals with type 1 diabetes. Manufactured by Medtronic, a global leader in medical technology, the system integrates a smart insulin pump with continuous glucose monitoring (CGM) to deliver precise, automated insulin doses. Medtronic has been at the forefront of medical innovation for over 70 years, providing trusted solutions in diabetes care. As of 2025, the system also has a U.S. indication for adults with insulin-requiring type 2 diabetes, expanding who can benefit from automation.
Specifications
- Size and Weight: Compact and lightweight for easy portability.
- Cartridge Size: Holds up to 300 units of insulin, suitable for extended use.
- Basal Rates: Fully customizable to individual needs.
- Bolus Delivery: Precise dosing for carbohydrate intake and real-time glucose levels, including automated correction boluses when needed.
- Water Resistance: Rated IPX8, enabling immersion in water up to 12 feet for 24 hours.
How the Minimed 780G System Delivers Insulin
The system uses Medtronic’s SmartGuard™ technology to automatically adjust insulin delivery every five minutes based on CGM readings. This automation helps users stay within their glucose targets, reducing the risk of both highs (hyperglycemia) and lows (hypoglycemia). Additionally, its Meal Detection™ feature can automatically deliver correction doses if meal boluses are missed or underestimated. These “auto-corrections” occur about every five minutes and are influenced by user settings such as target glucose and Active Insulin Time (AIT).
The Science Behind the Algorithm
The hybrid closed-loop algorithm powering the MiniMed™ 780G System was developed by Medtronic with input from extensive clinical trials and real-world users. It adapts to the unique needs of each user by continuously analyzing CGM trends, recent insulin delivery patterns, and carbohydrate entries. In practice, selecting an appropriate target (see below) and AIT (commonly 2–8 hours) meaningfully affects how assertively the system corrects highs after meals.
Target Glucose Levels
The 780G allows users to set a glucose target as low as 100 mg/dL (5.6 mmol/L). Additional target options include 110 mg/dL and 120 mg/dL. These can be adjusted with your care team to balance tight control and hypoglycemia risk. For physical activities, users can choose the 120 mg/dL target or other strategies to help maintain safe glucose levels during exercise. The AIT setting (typically 2–8 hours) also influences how often and how strongly the system gives auto-corrections.
FDA Approval
The MiniMed 780G received FDA clearance in April 2023 for individuals aged 7 years and older with type 1 diabetes. In 2025, the U.S. indication expanded to include adults (18+) with insulin-requiring type 2 diabetes. Availability and indications may vary by region; check local labeling and discuss with your healthcare provider.
What Comes with the Minimed 780G System?
When purchasing the system, users receive:
- The MiniMed 780G insulin pump
- Guardian 4 CGM and transmitter
- Infusion sets
- User guides and comprehensive training resources to ensure confident usage.
Infusion Set
CGM Compatibility
The MiniMed 780G is compatible with the Guardian™ 4 CGM. In 2025, Medtronic also announced and received U.S. clearances enabling additional sensor options: the Simplera Sync™ patch iCGM for 780G and a pathway for integration with Abbott’s Instinct™ sensor designed explicitly for MiniMed integration. Availability may depend on market rollout and labeling; check with Medtronic and your clinic for the most current options.
Mobile Apps for Control and Monitoring
The MiniMed™ Mobile app allows users to monitor glucose levels, pump data, and insulin information via smartphones and smartwatches. The CareLink™ Connect app provides caregivers and healthcare providers with remote access to the user’s glucose and insulin delivery data for seamless support (up to five care partners).
Battery and Maintenance
The system features a rechargeable battery that lasts several days on a single charge, making it convenient for daily use. Users should charge the pump regularly to ensure uninterrupted functionality.
Pros and Cons
Pros:
- automated insulin adjustments, including auto-corrections
- customizable glucose targets and adjustable AIT
- real-time monitoring features and remote sharing
- 7-day infusion set option reduces change burden
- water-resistant design
Cons:
- Some users note that regular maintenance, such as changing sensors and infusion sets, is required.
- Initial setup may feel overwhelming.
The Medtronic MiniMed 780G System represents a transformative step in diabetes management, offering advanced automation and customization to meet individual needs. For people with type 1 diabetes—and now many adults with insulin-requiring type 2 diabetes—this system can provide peace of mind and improved glucose control. To learn more, visit Medtronic’s official website or consult with your healthcare provider.
Explore AID Systems: CamAPS FX | DBLG1 | iLet | MiniMed 780G | Omnipod 5 | Sequel Twiist | Tandem Mobi | Tandem t:slim X2 | mylife YpsoPump | DIY Systems
Last Updated on January 28, 2026


