Automated insulin delivery (AID, artificial pancreas) systemsare progressing rapidly to reduce the burden of diabetes care. AIDs combine three to four components: an insulin pump, a CGM, a software control algorithm, and sometimes a cell phone app or dedicated PDA for convenient remote operation.
An AIDs software algorithm or “brain” can reside in the pump or pod, in a locked-down PDA, or as an app. Apps can reside in a cell phone or a PDA for more discrete operation. The algorithm oversees insulin doses tht regulate glucose levels amidst the daily impact of meals, exercise, stress, medications, hormone levels, and other factors.
AIDs lower or totally stop basal delivery for a predicted low glucose level and increase insulin delivery in spurts when the glucose is trending high. The wearer must still choose what bolus dose they require to offset the glucose rise from meals and snacks. A willingness to learn how to adjust pump settings and insulin doses for optimal outcomes is very helpful.
Glossary of Pump Lingo
- ACE—alternate controller enabled insulin pump
- AID—Automated Insulin Delivery or hybrid closed loop
- APS—Artificial pancreas system
- Basal—all day steady release of insulin
- BLE—Bluetooth Low Energy
- Bolus—a quick insulin infusion with carb boluses covering carbs and correction boluses lowering high BGs
- Bolus Calculator (BC)—calculates bolus dose to recommend
- CGM—continuous glucose monitor
- COB—carbs on board or undigested carbs still raising the glucose
- Correction Target—the BG a correction bolus aims for
- DIY—do it yourself
- Duration of Insulin Action (DIA)—how long a bolus lowers the BG—used to calculate residual IOB or BOB activity
- FDA—US Food and Drug Administration
- HCL—hybrid closed loop requires manual boluses along with helpful automation
- IOB or BOB—insulin on board or bolus on board still actively lowering the glucose from recent boluses
- iPump—interoperable pump, AKA ACE insulin pump
- MDI—multiple daily injections
- MPC—model predictive control algorithm
- PDA—personal digital assistant or dedicated phone
- PID—proportional integral derivative algorithm
- TIR—time in range, percentage of time your glucose is between 70 and 180 mg/dL (3.9 and 10 mmol)
- TDD—total daily dose—an average of all basal and bolus doses of insulin
AIDs provide significant benefits:
- Better glucose levels,
- Less frequent and severe hypoglycemia,
- More time spent in the target glucose range of 70 to 180 mg/dL (3.9 to 10 mmol/L),
- Less time spent below the target range, and
- A better quality of life with fewer burdens.
In handling AID risks, the FDA balances internal AID risks against the significant daily risks and problems faced by those without an AID. Annoyances can sometimes arise in bringing about better glucose values because of excessive alarms and alerts, finger-stick requirements for older CGMs, and reduced control over settings and glucose targets. AID wearers may also gradually unlearn the details needed to self-manage their glucose levels when an AID fails or becomes unavailable.
Anatomy of an AID
Control algorithms deliver short spurts of insulin based on various factors. Sometimes called “micro boluses,” these spurts are short-term decreases or increases in the basal rate when the projected glucose is going low or high, respectively.
Factors that control insulin delivery differ between algorithms. To determine doses, a system may rely on the current TDD, current basal rate, estimated fasting insulin level, the recent history of doses and glucose readings, or recent and projected CGM readings. Insulin delivery is often limited as a maximum multiple of current basal rate entries.
When data is downloaded, insulin delivery appears as small basal-constrained “corrective boxes” of insulin, varying in height between zero (no delivery) to the max-multiple of the basal rate that the system or setting allows. Basal modifications appear alongside the units of meal and correction doses at the time given.
Predicted glucose values rely on current CGM glucose readings, the trend line, the expected rise in glucose from recent carb intake, and any glucose reduction expected from the residual insulin on board (IOB). IOB is determined by the time entry in the duration of insulin action setting (DIA).
The DIA or insulin action time (IAT) becomes a critical setting because it determines the estimated IOB. Short DIA times hide insulin stacking and can become serious if an insulin buildup exceeds an AID’s ability to reduce insulin through suspension of the basal rate. A short DIA time blinds the pump wearer (and their pump or AID) to how many insulin units are still actively lowering the glucose. In addition, it becomes impossible to determine how many grams of carb you really need to treat a low glucose.
An AID can detect and predict glucose changes more effectively once it knows how many units of IOB remain active. A correct DIA time allows the algorithm to determine how many units of insulin remain to lower the glucose. This helps reduce insulin stacking and hypoglycemia. In addition, an accurate DIA time allows other pump settings to be appropriately optimized.
JDRF and FDA Step Up to the Fast Pace of Diabetes Technology
Interest in AID devices is quite high. The market share in 2017 of $90 million is expected to grow to $279 million by 2024.1 While encouraging, advances in medical devices and software for treatment often outpace insurance company policies that allow a pump or CGM to be replaced only every 4 to 5 years.
Historically, wearers have been often stuck with outdated equipment for months or years until their warranty allows them to replace the entire system. A new component may not work with older components and owners are constrained to one manufacturer when others offer improved technology.
Interoperable and Interchangeable Devices
To address these issues, the Juvenile Diabetes Research Foundation (JDRF) encouraged the FDA in August 2017 to consider an Open Protocols Initiative. The OPI allows plug-and-play AID components to be interchanged. Users would have more choices and better access.
In March 2018, the FDA gave Dexcom’s G6 CGM its first plug-and-play iCGM designation. This allowed G6 CGMs to share glucose data with any other device that receives an interoperable designation. To get an iCGM designation, a CGM must show sufficient accuracy and work interactively with any iPump or iAlgorithm through BLE communication.
Interoperable devices became possible in 2010 when the 4th version of Bluetooth, called Low Energy, or BLE, was released. BLE enabled secure, reliable communication between nearby devices over greater distances with less demand on batteries than the older versions of Bluetooth. Bluetooth devied its name from King Harald “Bluetooth” Gormsson who unified warring factions in 10th century Denmark.
In October 2018, the FDA also introduced the alternate controller enabled (ACE) designation for insulin pumps. Often referred to as an iPump, the ACE designation assures delivery accuracy, reliability, cybersecurity, and secure communication with compatible iCGMs and iAlgorithms.
These changes allow manufacturers to integrate new technologies or software with existing technologies with an interoperable designation. This significantly reduces the clinical research burden and costs for devices. A new iCGM can work with any existing iPump or iAlgorithm. For example, an older iPump could now download software allowing it to work with a newly released iCGM or iAlgorithm.
Be Careful if You Take an SGLT-2 on an AID!
Most diabetes medications can be used with an AID. However, SGLT-2s, like Jardience and Farxiga, pose risks. SGLT-2s work by passing excess glucose into the urine. An AID normally increases insulin delivery to lower high glucose readings. However, as the SGLT-2 lowers the glucose, an insulin deficit can become severe with less elevation in the glucose.
Taking an SGLT-2 makes ketoacidosis harder to identify while wearing an AID, such as when an infusion set starts to leak insulin or gets detached. The FDA does approve these meds for Type 1 diabetes. Choose either an AID or SGLT-2, preferably not both.
Interoperable Devices
The FDA details the studies and data that a pump, CGM, or algorithm requires to get the interoperable designation. Companies have widely welcomed this regulatory move. This allows them to more rapidly develop and deliver innovations to the diabetes community, knowing they will work with existing devices. Plug-and-play upgrades to new CGMs and control algorithms are expected to lower costs and enable faster insurance coverage.
The devices below have been at the forefront of interoperable plug and play devices for diabetes:
Dexcom—iCGM
In March 2018, Dexcom received the FDA’s first designation for an interoperable device with its G6 CGM or iCGM after passing strict accuracy requirements. The G6 sensor comes factory calibrated, so finger sticks are not required. You can also calibrate the G6 if a meter reading or two disagrees with the CGM’s reading.
Readings can be shared with up to 10 people, and users can get vocal reports of current glucose readings from Siri. Dexcom’s next-generation 14-day G7 sensor is expected to be disposable, smaller, and available at lower cost.
Senseonics and Medtronic are also working on iCGM designations for an Eversense implanted CGM and a new Zeus CGM, respectively. Agamatrix, Medtrum, and Sooil, among others, are also working on new iCGMs.
Tandem Diabetes—ACE iPump
On Valentine’s Day, 2019, Tandem’s t:slim became the first pump to get the FDA’s new ACE (alternate controller enabled) infusion pump or iPump designation, partly because of their early adoption of BLE communication. Bigfoot and Sooil have also integrated BLE in their current pumps. Tandem has another BLE pump in development, and Omnipod has BLE in their current DASH pod. Medtronic has two new pumps with BLE, now undergoing testing.
Tandem Diabetes—iController and First Fully Interoperable Closed Loop
On December 13, 2019, Tandem’s Control-IQ software received the first “iController” status for its fully interoperable AID system. Combined with Dexcom’s G6 iCGM, the Tandem t:slim X2 iPump, and Tandem’s Control-IQ iAlgorithm, this became the first interoperable AID system.
DreaMed Diabetes, an Israeli company developing personalized diabetes management solutions, has received FDA approval for its Advisor Pro fuzzy logic/artificial intelligence algorithm. This guide for insulin dosing decisions by healthcare professionals may be integrated by Medtronic into its AIDs. Medtronic is also working to improve its 780 pump PID algorithm using Tidepool’s Loop MPC algorithm. FDA approval is pending. Neither has iController status at this time.
Tidepool Loop
Tidepool, a 2013 Palo Alto diabetes data startup, is seeking FDA approval for a commercial version of the open-source DIY Loop AID algorithm. Tidepool Loop works with commercial Bluetooth insulin pumps, CGMs, and an iPhone or Apple Watch. The Loop algorithm is currently used by thousands of Type 1s, potentially speeding FDA approval.
In May 2019, the FDA issued a warning about DIY Artificial Pancreas Systems after one user experienced an accidental insulin overdose. Accidents can happen with any pump or AID. However, reports of failures to the FDA remain sketchy with no regulatory body tracking failures, especially between different systems.
Pete Schwamb and Katie DiSimone helped develop Loop’s DIY algorithn and online documentation. Tidepool hired them to aid production of a commercial version. Omnipod and Medtronic are simultaneously pursuing their own new control algorithms, and have signed up to use the Tidepool Loop iAlgorithm, once approved.
TypeZero
Dexcom acquired TypeZero Technologies’ inControl Diabetes Management Platform in August 2018. Univ. of Virginia researchers developed this AID algorithm and it is undergoing extensive testing.
The AID Comparison Table ate bottom shows the variety of companies with already approved iPumps, iCGMs, and iAlgorithms, along with those under development.
Training Needed for Users and Clinicians
Many pump wearers switch to AIDs using the same or similar settings to those on their insulin pumps. Unfortunately, pump setting errors are rampant, as demonstrated by the poor A1c results and excessive glucose varibility among pump wearers. Setting errors generate erroneous basal and bolus doses and, unless remedied, place those who start on an AID at a serious disadvantage. Training for clinicians and users is lacking.
Clinicians and users are also often unaware of which settings will even impact their glucose control after starting on an AID. Which setting should they change and by how much when readings remain erratic? Which have no relevance in the algorithm? Additional training and guidance are required for clinicians and users. We encourage you to visit our Pump Dose Guide for setting suggestions before starting on an AID or when attempting to salvage an inauspicious start. (Can be quite helpful for those on MDI as well.)
Current Status of Commercial and DIY AID Systems
Medtronic 770G
Approved by the FDA in 2016 and being first on the market, the Medtronic 670G was designed with conservative software to get FDA approval and protect against lawsuits. This resulted in excess time spent out of AutoMode and alarm exhaustion for many users, with a significant number turning off the alarms or abandoning AutoMode entirely.
The newer 770G available in the U.S., and the 780G available in Europe and the Middle East, have improved sanity and sleep. Similar to the 670G, the 770G remains a reliable system with an outmoded design. It benefits from fewer alarms and more time spent in AutoMode. AutoMode especially benefits those who start with appropriate pump settings, bolus before meals, and calibrate the CGM at bedtime to avoid a middle-of-the-night calibration alarm.
The 780G has a newer algorithm that adds small correction boluses to increased basal delivery for high glucose readings. In addition, its glucose target can be set as low as 100 mg/dL, and Bluetooth connectivity enables software updates. Both the 770G and 780G systems rely on the Guardian 3 seven-day sensor that requires twice-a-day calibrations. The 780G will integrate a newer CGM awaiting approval that may require only one calibration on day one of wear.
In AutoMode, basal delivery and correction boluses are largely determined by recent TDDs and estimated fasting insulin levels. To regulate glucose levels, users can modify values for insulin action time (IAT or DIA) or the insulin:carb ratio. Users are sometimes encouraged to lower their IAT (DIA) to reduce elevated glucose levels. However, this can result in “unexplained” hypoglycemia caused by the larger bolus doses this generates. If glucose levels are routinely elevated, better advice is to lower the I:C number and be sure to bolus before every meal using an accurate ICR and carb count
AutoMode is intended to minimize episodes of glucose going below 70 mg/dL (3.9 mmol/L). The fixed glucose target of 120 mg/dL (6.7 mmol/L) in AutoMode ensures against hypoglycemia. For increased activity, the user can select an exercise glucose target fixed at 150 mg/dL (8.3 mmol/L). To be effective, start the exercise target at least 2 to 3 hours prior to any serious increase in activity. You might still need some carb intake during exercise, but not nearly as much. If your activity is increased or decreased for 24 hours or more, AutoMode can take a few days to adjust because doses are determined from the previous 6-days of history.
Tandem Diabetes Control-IQ
Tandem Diabetes integrated its Tandem Device Updater software into the t:slim pump in late 2016. This enables convenient software upgrades over the Internet without the need to replace the pump itself.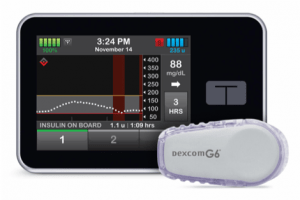
Tandem’s full Control-IQ AID system became the first interoperable software for AIDs. Tandem’s interoperability allows it to work with Dexcom’s current G6 iCGM. It is also expected to work with Abbott’s pending Libre 3 iCGM. People wearing the t:slim X2 pump can download Control-IQ into their pump for free by signing into their account, request a prescription from their health care provider, and then pass an online training session in the system’s features and use.
In Control-IQ, the wearer can adjust basal rates, I:C ratios, and correction factors to improve control. The DIA time is preset to 5 hours for insulin action to minimize insulin stacking. This is safer and saner than systems that allow short DIA time entries that do not match the physiologic action of current pump insulins in the body.
The Control-IQ algorithm improves time in target by aiming to keep daytime glucose values between 112.5 and 160 mg/dL. Basal delivery gets reduced if the glucose is projected to go below 112.5 mg/dL (6.3 mmol/L) to lessen hypoglycemia, while insulin micro-bursts are increased once the glucose is projected to go above 160 mg/dL (8.9 mmol/L). If the glucose is projected to go above 180 mg/dL (10 mmol/L), small correction boluses, equal to 60% of the expected correction bolus with a target of 110 mg/dL (6.1 mmol/L), are added each hour on top of the micro-bursts. Thus, an accurate CorrF or ISF becomes important.
During sleep, a lower glucose target of 110 mg/dL can be selected as Sleep Mode by the user. Some experienced users select Sleep Mode for 24-hours to lower their average glucose. For exercise, Control-IQ has an option to keep the glucose between 140 and 160 mg/dL (7.8 and 8.9 mmol/L). Without relying as Medtronic does on the last 6-days glucose history, the glucose levels adjust rapidly for major changes in activity, such as weekend warriors or starting or stopping marathon training. An alternate profile for exercise is easy to do in this pump, such as lower basal rates and higher carb and correction factors for exercise. Activating the profile allows rapid accommodation for heavy periods of activity or exercise.
You must still bolus before meals. However, if you forget a meal bolus, Control-IQ detects the rising glucose and automatically delivers about 60% of the usual meal bolus dose using a glucose target of 112.5 mg/dL (6.3 mmol/L).
In a pivotal research study, 168 participants using the Tandem Control-IQ system spent 2.6 more hours a day in the 70-180 mg/dL (3.9-10 mmol/L) target range and lowered their average A1c by 0.3% from 7.4% to 7.1% compared to a control group with the same pump and CGM but no automation. Everyone completed the study with 92% of the full six-month time period spent active in AID and 71% of this time spent in time in range.
On a 5-point scale, participants in one study rated ease of use at 4.7 out of 5, usefulness at 4.6, trust at 4.5, and desire to continue its use at an impressive 4.8. User’s reasons included simplicity, no finger sticks with the Dexcom G6 CGM, near-continuous automated operation, and a minimal number of alarms. Clinicians conducting this Phase 3 trial reported that none of the people using this system wanted to discontinue its use.
Insulet Omnipod 5 AID
Insulet became the 2nd company to receive FDA’s ACE designation in September 2019, for its Omnipod Dash system. Dash meets the FDA’s cybersecurity, reliable communication, design, and transparency requirements. This ensures it can reliably and securely communicate with other Bluetooth-enabled devices, as well as AID software, and that it can receive, execute, and confirm commands from these devices.
Insulet’s new Omnipod 5 AID has been submitted to the FDA. It will communicate with Dexcom’s G6 iCGM or the new G7 iCGM, and eventually with the Freestyle Libre 3 CGM that is seeking iCGM status. Insulet is working on two hybrid closed-loop algorithms, its own internal Horizon MPC algorithm and, a partnership with Tidepool, on Loop’s DIY algorithm that may be close to FDA approval. We expect both to have smartphone app controls.
A Loop DIY version for Omnipod Eros pods is already available.
DIY AID Systems
Although not FDA-approved, open-source DIY AID systems continue to be available because of their non-profit status. A few thousand people use DIY systems. With no federal oversight, these systems do not undergo traditional clinical trial processes and standards and bypass the slower paths required of corporations for FDA approval.
The ease of use of DIY systems has improved. Costs vary from $0 for some Omnipod or Dana users, to $150 for a communication module for someone who has an older Medtronic pump, to as much as $1,000 for someone getting both the controller and an older Medtronic pump. In theory, DIY systems might improve A1c results with fewer highs and lows and more time in the desired range than commercial systems. However, the user is responsible for some daunting programming, and the selection of settings, targets, and alarms.
OpenAPS
The DIY movement pioneered interoperability. Dana Lewis, Scott Leibrand, and Ben West started the movement in 2014, using Linux software on a small Raspberry Pi computer. A communication module was developed for radio communication with older Medtronic pumps and direct BLE communication with other devices. Other motivated and organized individuals and parents quickly joined their open-source effort. The current system uses a dedicated circuit board or an app on some Android phones that communicate with some Dana insulin pumps and Omnipods, as well as Dexcom and other CGMs.(#WeAreNotWaiting)
OpenAPS offers a “carbs required” alert when the projected decrease in basal rates may not be sufficient to prevent a low glucose. Originally offered in the Deltec Cozmo pump in the early 2000s, all AIDs should incorporate this option. Distinct from Loop and AndroidAPS below, meal boluses are delivered through the pump bolus calculator rather than a smartphone. Also available are Autotune and Autosens that transfer the day’s data at night to a Nightscount account for suggested changes in pump settings the next day.
Loop
Loop DIY systems are based on an open-source algorithm that uses Riley Link hardware ($150) with a communication board and rechargeable battery in a plastic case. Components often include a Dexcom CGM and an older Medtronic pump (See Figure 1)

Like all DIY systems, it helps to be tech-savvy or have friends who are to personalize the pump, phone, and software. Steps include becoming an Apple developer (free or $99 per year), using Xcode software from Apple for programming (free), and potentially encountering programming errors for those first trying Xcode. For programming and setting change issues, a large online user manual details each step, and online chat groups and blogs provide lots of help.
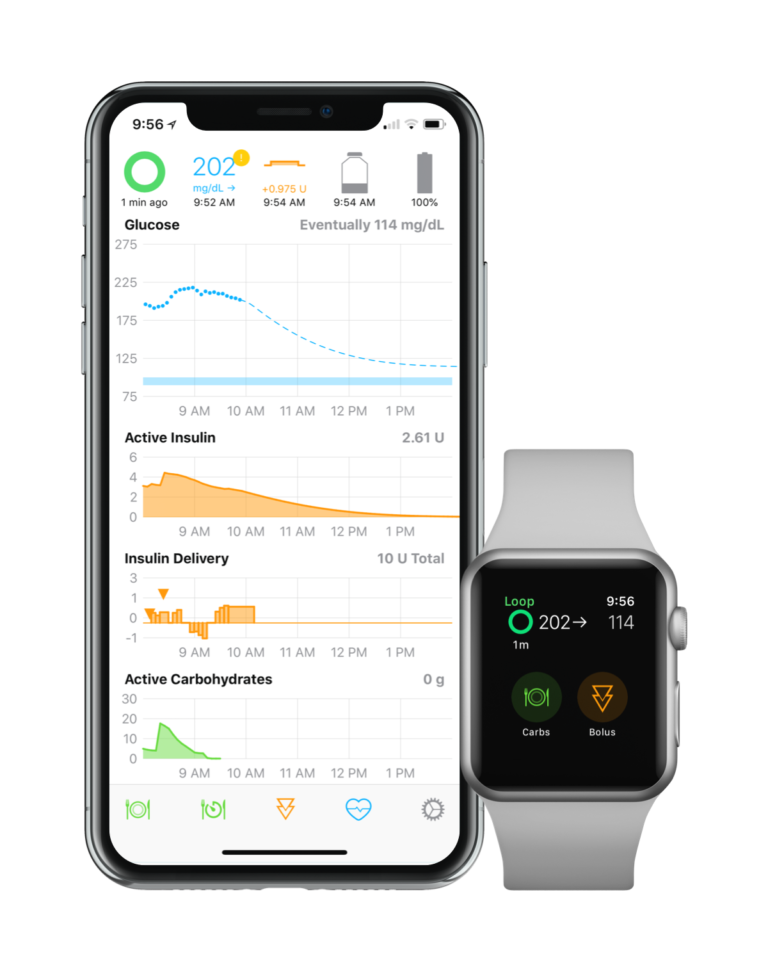 Basal and bolus settings, BG targets, and carbs on board (carb digestion that is still raising the glucose) are adjusted through a Loop app on an iPhone 6 or later. No correction bolus is automatically given for elevated glucose readings, but Loop compensates by allowing the user to set its maximum basal rate at high values. This enables larger “correction bursts” over short time intervals. The user can carefully adjust their max basal rate setting upward to more aggressively correct elevated glucose readings once they’ve entered an accurate DIA time to avoid insulin stacking. Recommended settings for the maximum basal increase are 2 to 5 times the current basal setting. The Loop system tracks carbs on board (COB) and allows the user to set digestion times for fast, medium, and slow carbs when consumed, adding value to simple carb counting.
Basal and bolus settings, BG targets, and carbs on board (carb digestion that is still raising the glucose) are adjusted through a Loop app on an iPhone 6 or later. No correction bolus is automatically given for elevated glucose readings, but Loop compensates by allowing the user to set its maximum basal rate at high values. This enables larger “correction bursts” over short time intervals. The user can carefully adjust their max basal rate setting upward to more aggressively correct elevated glucose readings once they’ve entered an accurate DIA time to avoid insulin stacking. Recommended settings for the maximum basal increase are 2 to 5 times the current basal setting. The Loop system tracks carbs on board (COB) and allows the user to set digestion times for fast, medium, and slow carbs when consumed, adding value to simple carb counting.
The Nightscout open-source data-sharing project through Heroku enables access to an Autotune option for advice on improving glucose readings. Autotune looks back at several weeks of Nightscout data to suggest changes to the basal rates, CorrF/ISF, and CarbF/ICR. Setting changes are limited to 20 to 30% above or below the underlying pump values, so starting with appropriate settings speeds progress. We do not know how long it will take for commercial companies to integrate some version of an Autotune concept into future AIDs. Percentage Profile Switch lets the user adjust both basal rate and ISF for quick adaptations for rapid control shifts for illness, activity changes, etc.
AndroidAPS
Developed by Milos Kozak and Adrian Tappe in Europe, the AndroidAPS system works on Android phones with current pumps with BLE such as Dana R and RS and Roche Accu-chek Combo and Insight. Again, Nightscout through Heroku enables an Autotune option for advice on improving glucose readings.
Control Algorithms
Engineers have developed math-based approaches to handle complex situations in multiple arenas where predicting outcomes using current inputs leads to better results, such as with today’s commercial airliners and self-driving cars. Diabetes is a perfect example of an arena where this can be helpful. The two most commonly used algorithms are model predictive control (MPC) that is considered more proactive or predictive, and proportional-integral-derivative (PID) that is considered more reactive. Many varieties of each are in development. A recent review of research studies on control algorithms found that “MPC performed as well or better than PID in all metrics.” Most AIDs currently use MPC algorithms.
Model Predictive Control (MPC) algorithms are currently a favored design for advanced control systems in diabetes because their flexible design allows the system to handle multiple inputs through the use of quadratic equations. Accounting for things like exercise or pregnancy tends to be easier with MPC. Each input can then be optimized in a constrained way every time a prediction is made, such as every 5 minutes when new glucose arrives from the CGM.2 Insulin delivery is determined by its ability to reduce a forecast glucose level against the desired target glucose levels over the next 1.5 to 4 hours. Researchers have developed many variants of MPC for AIDs.3
An early MPC patent recommended safety constraints for AID systems.4 Their recommendations included setting the maximum basal rate increase no larger than 2 to 5 times the current basal rate for reduction of high glucose levels, full suspension of basal rates for glucose predicted to go below 77 mg/dL (4.3 mmol/L), and reduced basal delivery any time the glucose falls rapidly. They also recommended delivering only the current basal rate when an infusion set occlusion is inferred to more rapidly identify the real issue and minimize the introduction of false insulin delivery data into the algorithm, as the latter may introduce glucose turbulence and erode the accuracy of the mean TDD.
PID algorithms adjust insulin delivery by the rate of change in glucose (derivative), its deviation from a target glucose (proportional), and the area under the curve between the measured and target glucose (integral). PID responds to the current glucose rather than to a predicted glucose, such as predicted effects from meals or activity. Other algorithms, such as those based on fuzzy logic, are also being tested.
Other Developments
Other companies, like Insulet, Bigfoot Biomedical, Beta Bionics, Lilly Diabetes, and Roche, are in various stages of development and testing for AID systems. In addition, Diabeloop, a French company, has received the CE mark for distributing its AID in Europe.

One exception to insulin-only systems is Beta Bionics iLet. They have taken the lead on a dual hormone system that uses insulin to lower the glucose and glucagon to raise the glucose and more effectively guard against hypoglycemia. Zealand Pharma has developed dasiglucagon that is now in clinical trials with the Beta Bionics iLet pump, while Xeris is working on a similar trial with a glucagon solution for bi-hormonal AIDs at Oregon Health and Science University.
The Slow Path to Faster Pump Insulins
A major obstacle for AIDs involves the inherently slow action of current insulins compared to the faster digestion times of most foods. The total duration of insulin action with insulin release from a healthy pancreas is less than 40 minutes. In contrast, commercial insulins infused below the skin are slower to start and have a much longer DIA. DIAs are at least 5 hours in both children and adults.
Insulin released by the pancreas goes directly into the portal vein to the liver that processes a large portion of the glucose entering it. Subcutaneous delivery of Novolog, Humalog, and Apidra takes 20 to 40 minutes before the insulin starts to lower the glucose. By the time subcutaneous insulin reaches the liver, its levels are too low to activate the help of this crucial organ.
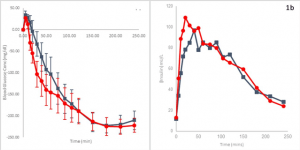
Some help has arrived with the release of Lilly’s “ultra-rapid” Lispro (URLi) insulin, called Lyumjev (LOOM-jev). Compared to Humalog (lispro), Lyumjev reduces glucose levels by 28 mg/dL in Type 1 subjects and by 12 mg/dL in Type 2 subjects at one hour and by 31 mg/dL in Type 1 subjects and by 17 mg/dL in Type 2 subjects two hours after a test meal. Lyumjev starts to lower the glucose by about 21 min. after being given, compared to 28 min. for Humalog. The overall impact appears good. In the graphic on the left in the figure below, at 50 minutes after injection, an elevated glucose level is lowered by 120 mg/dL with Lyumjev (red line). This compares to only 65 mg/dL with Humalog (blue line).
Now FDA-approved for pumps, users report the Lyumjev may be more stable in pumps, than Novo’s FiAsp that often loses potency on about day two. However, reports of loss of action and site issues are coming from pump wearers. The use of a Lyumjev pen for occasional high readings may be one solution. Keep in mind that the DIA for Lyumjev is minimally different from Humalog. It works as long as shown by the red line in the graph on the right. Its activity simply peaks earlier. A new formula for this faster decline in action would be helpful for pumps and AIDs.
To speed absorption, Lyumjev adds citrate for faster absorption and low concentrations of treprostinil (a prescription drug with potent vasodilator effects) that also enhances absorption to Humalog. Balance is critical: too much citrate speeds absorption but unfortunately also destabilizes the insulin. Treprostinil’s level allows local dilation of blood vessels without causing any systemic effects. Other standard ingredients include zinc, magnesium chloride, m-cresol, and glycerol.
Summary
New hybrid closed loops and AIDs will gradually reduce user burdens significantly. Our AID Comparison Table reviews advances in current and future systems in all areas.
| Comparison of Current and Future AID Systems | |||
|---|---|---|---|
| AID System | Components | Details | Approvals |
| Medtronic 670G | Guardian 3 CGM, PID algorithm | 180u or 300u reservoir, 2-4 calibrations a day, no remote monitoring, I:C ratio is the only control option, not capable of software upgrade, excess alarms for some users, too many exits out of AutoMode, BG target: 120. | CE: 2012 FDA: Nov, 2016, release in summer, 2017 |
| Medtronic 770G | Zeus CGM similar to Guardian 3, Tidepool commercial Loop MPC algorithm on iPhone, Riley Link. | BLE, easy software upgrades, calibrations unclear, multiple control options, remote monitoring, BG target selected by user. Loop usually works with BLE CGMs like Dexcom – unclear if this will occur. | FDA: pump and iCGM 1st half 2020? |
| Medtronic 780G | Synergy disposable CGM, updated PID or DreaMed Advisor Pro fuzzy logic algorithm. | BLE, easy software upgrades, no calibrations, user control options?, remote monitoring, BG target: 100 to 120 (150 for exercise), applying for iCGM designation, 7-day infusion set. | FDA: iCGM and AID 2ndhalf 2020 or 2021
FDA approved DreaMed Advisor Pro in Sept 2019 |
| Tandem t:slim | Dexcom G6 or Abbott Libre 2 iCGM,
TypeZero inControl MPC algorithm,locked-down PDA or phone app |
Color touchscreen, no calibrations, multiple control options, remote monitoring, always stays in AID, few alarms, auto basal shut off for predicted lows, basal increase above 160 mg/dL, hourly auto 60% of expected correction bolus minus IOB above 180 mg/dL, 300u reservoir | FDA: iPump ACE approval Feb 2019
FDA: Control-IQ now available and shipping |
| Tandem t:sport | Dexcom G6 or G7 iCGM, TypeZero inControl MPC algorithm, locked-down PDA or phone app | Color touchscreen PDA, no calibrations, multiple control options, integrated bolus button, patch or pocket/belt wear, easy software upgrades. Target: 112.5-160 mg/dL daytime, 112.5-120 mg/dL overnight, operates independently of PDA, 200u reservoir | FDA: 2ndhalf of 2020 or early 2021? |
| Loop DIY | Dexcom G5 or G6 CGM, Old Medtronic pump or current Omnipod, OpenAPS, RileyLink BLE and radio relay box, Loop MPC app on iPhone or iWatch | Xcode software, fully customizable, alerts only for good reason, no calibrations with G6, multiple control options, remote monitoring, 180 or 300u reservoir, requires technical skill, BG target set by the user, Autotune | Non-FDA, available now: $150 for Loop, $99 for Apple developer license. |
| OpenAPS DIY | Dexcom G5 or G6 CGM, old Medtronic pump, Linux on a Raspberry Pi microcomputer, Open APS MPC algorithm | Linux software, fully customizable, alerts only for good reason, no calibrations with G6, multiple control options, remote monitoring, 2 or more profiles, Autosens settings guide, 300u reservoir, requires technical skill, BG target set by the user | Non-FDA, available now. |
| AndroidAPS DIY | Dexcom G5 or G6 CGM, BLE-enabled Dana R or RS pump or Roche Combo or Insight pump, AndroidAPS MPC software, Ruffy app, and LineageOS or Android 8.1 on phone | Android Studio software, fully customizable, alerts only for good reason, no calibrations with G6, multiple control options, remote monitoring, 2 or more profiles, Percentage Profile Switch lets the user adjust both basal rate and ISF for quick adaptation, 300u reservoir, requires technical skill, BG target set by the user | Non-FDA, available now. |
| Insulet Omnipod Loop | Dexcom G6 or G7 iCGM, Tidepool Loop app on iPhone, Tidepool Loop MPC algorithm | Color touchscreen PDA or app on select Samsung phones, pod can operate independent of PDA, no calibrations, multiple control options, remote monitoring, 200u reservoir, BG target set by the user, (39mm x 52mm x 14.5mm) | FDA: iPump ACE approval Sept 2019
FDA: Full system 1s thalf 2020? |
| Insulet Omnipod Horizon | Eros pod, DASH, locked-down color Sansum Android PDA, Dexcom G6 or G7, U of Virg.TypeZero inControl MPC algorithm | DASH locked-down color touchscreen Samsung phone, easy software upgrades, no calibrations, remote monitoring, multiple control options, 200u reservoir, pod can operate independent of PDA, BG target: ? | FDA: iPump approval Sept 2019
FDA: Full system 2nd half 2020? |
| Bigfoot Biomedical SmartLoop | Loop (Asante Snap) BLE insulin pump, Freestyle Libre 2 CGM, Tidepool Loop or perhaps TypeZero inControl MPC algorithm, phone app | Low starting price via monthly subscription, easy software upgrades, remote monitoring, 300u pen fill easy cartridge change, no calibrations?, multiple control options, BG target: ? Bigfoot was first to receive FDA’s fast track approval for its AID in November 2017. | FDA: 2019 or 2020
with Dexcom’s iCGM and iAlgorithm ACE designation for Snap iPump 1sthalf 2020? |
| Beta Bionics iLet Bionic Pancreas | iLet bi-hormonal pump, Dexcom G6 or Senseonics CGM, Gen 4 Touchscreen PDA, PID algorithm | No or few calibrations, multiple control options, remote monitoring, BG target: 120 mg/dL for insulin, 110 mg/dL for insulin/glucagon | FDA: 2020 for insulin only,
2021 for insulin + glucagon |
| Roche Diagnostic Ltd.
|
Accu-Chek Insight pump or Solo patch pump? DexCom G6 iCGM or Sensonics Eversense CGM, TypeZero Technologies inControl MPC algorithm
|
2019 International Diabetes Foundation Closed-Loop research study involved 43 subjects at 14 sites in the US and Europe | CE: 2020?
FDA: 2021? Roche remains quiet in this area |
| Diabeloop DGLB1 System | Wearable Kaleido patch pump with short infusion set, locked-down Sony Xperia Z1 PDA, Dexcom G6 CGM | No calibrations, multiple control options, remote monitoring, 200u reservoir, BG target: 100 to 130 mg/dL | CE: 2019
FDA: 2020? |
| Lilly Diabetes | Lilly Deka (Dean Kamen) 2” diam disk pump, Dexcom G6 or G7, McGill Univ. algorithm, dedicated controller or phone app, 3 ml reservoir | Reusable and disposable components, patch or pocket/belt wear, no calibrations, multiple control options, remote monitoring, use any luer lock infusion set, 200u reservoir, BG target: ? | FDA: 2020 or 2021? |
| SFC Fluidics | Hydraulic pump with 2-way safety valve and Percusense CGM housed in a single on-body pod, Diabeloop machine-learning algorithm, JDRF funded | Disposable patch pump? | FDA: 2020 or 2021? |
| Agamatrix | Agamatrix iPump and Subsidiary Waveform Technologies’ iCGM, Oregon Health & Science Univ. MPC algorithm, Apple or Android phone app
|
One hour warm-up, glucose reports each minute, 14-day sensor wear, calibrations?, user options?, BG target: ? mg/dL | FDA approval late 2019 for Waveform iCGM?
2020 or 2021 for iPump?
|
| Medtrum | P6 EasyPatch disposable pump, S7 EasySense CGM, A6 TouchCare System with predictive low BG Suspend | Limited usage in Europe, rechargeable touchscreen PDA for pump control, colored “brain” part of pod is reused, 200u reservoir in white section is reused, lower cost monthly rental, integrated 5 mm 30 gauge stainless steel needle, 3-day wear | CE: 2019
FDA: 2020 for pump? Unclear if S7 CGM has accuracy for iCGM status |
| Sooil Dana | Dana Diabecare RS BLE pump, OpenAPS MPC algorithm, Dexcom G5 or G6 CGM, Apple or Android phone | 300u reservoir, BG target set by the user, full user calibrations, multiple control options, remote monitoring, only one proprietary reverse-luer-lock Teflon infusion set. | FDA: 2020? |
| EOFlow EOPancreas | EOPatch pump (9.9mm x 32.4mm x 12.9mm); EOCloud algorithm derived from early Type Zero software? Locked-down Android phone or Android app. | Disposable, CGM in a pod under development, 200u reservoir, integrated 30 gauge stainless steel needle, calibrations likely, multiple control options, remote monitoring, 3-day wear. EOFlow received fast-track approval from the FDA for its AID in March 2019. | FDA: pump 2020? AID 2021? Unclear if CGM has accuracy for iCGM status |
Suggestions to Improve AID Systems
- Train HCPs and users in the critical details required to succeed with each of these complex glucose management systems.
- Manufacturer clearly states how each pump setting impacts the user’s glucose outcomes and how different settings interact. Will a low basal rate be a handicap if microbursts are limited to a multiple of this rate? Which settings and what circumstances determine the maximum correction dose? What benefits and risks arise from the use of short DIA (IAT) times?
- Allow two or more sets of settings: one for normal pump operation during CGM changes, etc., another with more aggressive settings for use during AID operation, another for team sports during the school year, etc.
- Provide an alarm for infusion set failure since this continues to be the most vulnerable component in AID systems.
- Track Carbs on Board with the user able to set duration of carb action times for fast, medium, and slow carbs (Loop and AndroidAPS have this).
- If the AID cannot reduce the basal rate sufficiently to prevent hypoglycemia, the system should inform the user exactly how many grams of carb they need to prevent the pending hypoglycemia (the Deltec Cozmo insulin pump did this over a decade ago).
- Include the user’s weight as a setting for accurate estimates of the insulin to carb ratio and determination of the exact number of carbs needed to treat each low or pending low glucose.
- Allow the user or clinician to individualize the glucose targets once they document hypoglycemia risk to be minimal.
- Once 14 days of glucose data are available, the AID system should provide common solutions for any pattern of hypo or hyperglycemia.
- Through human factor studies, confirm that a new user can take everything out of the delivery box, select initial personalized settings, and safely start on the AID on their own.
- Integrate activity monitors for rapid adjustments in insulin delivery for seasonal sports or a new sport or a change in work activities.
Written by John Walsh, PA, CDTC, and Ruth Roberts, MA
1 Coherent Market Insights. “Artificial Pancreas Device System Market Size Is Projected to Reach USD 341 Million at a CAGR of 21.1% By 2023” heraldkeeper, 01 January 2019, http://heraldkeeper.com/news/artificial-pancreas-device-system-market-size-is-projected-to-reach-usd-341-million-at-a-cagr-of-21-1-by-2023-473567.html
2 B. Wayne Bequette, Ph.D. Algorithms for a Closed-Loop Artificial Pancreas: The Case for Model Predictive Control.
3 The Artificial Pancreas: Current Situation and Future Directions. Edited by Sánchez-Peña RS and Cherñavvsky DR.
4 Wilinska ME, Budiman ES, Hayter GA, Taub MB, and Hovorka R. Integrated closed-loop medication delivery with error model and safety check. US Patent 9,402,953 B, 2016.
