Insulin pump companies are producing better and better pumps. There are many similarities when comparing insulin pumps but there are some important differences, like the differences in calculating Bolus on Board or which infusion sets can be used. This is the current batch of insulin pumps that are available:
| One Touch Ping Insulin Pump by Animas Corporation 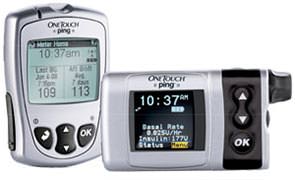 |
t:slim X2 Insulin Pump with Basal IQ by Tandem Diabetes 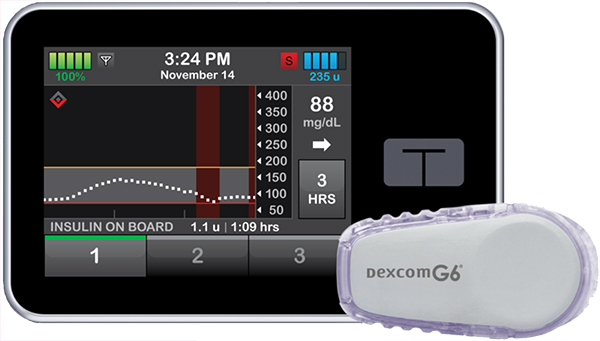 |
| 670G by Medtronic Minimed 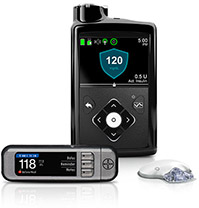 |
Accu-Chek Spirit Combo Insulin Pump by Accu-Chek Insulin Delivery Systems  |
| Omnipod Insulin Pump by Insulet  |
Snap Insulin Pump by Asante 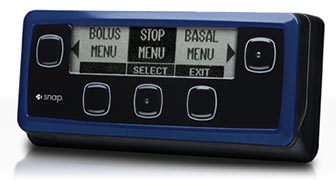 |
| Dana Diabecare IIS Insulin Pump by Sooil  |
